How to make an economic evaluation of vaccination plans against Escherichia coli mastitis?
We are launching a series of 3 new posts to focus on a key aspect of decision making on a farm: the economic return. In this case, we are going to learn, from Oriol Franquesa Oller – Qllet SLP, what aspects to assess for the creation of a starting scenario, and how and for how long we should evaluate the economic return.
Introduction
Costs related to clinical mastitis and milk quality have been a source of concern for dairy farms for many years. Much effort has therefore been dedicated to improving the prevention of this disease, which has involved the control of contagious microorganisms, improving the conditions and hygiene of the facilities where animals live, improving the milking routine and the training of workers, and improving the treatment of cases of clinical mastitis. In recent years, this final point has been of particular importance, in relation to the reduction of resistance and the responsible use of these antibiotics.
Among the resources used for these purposes, we also have vaccines for the prevention of clinical mastitis.
The use of vaccines for the prevention of mastitis is not new. The first vaccines with the J5 component for the prevention of mastitis caused by E. coli have been present on the American market since the 1980s.
“However, the farmer’s recurrent question is always the same: what economic return will I get with this product?”
The answer is not always easy. It depends on several important factors:
- First, the farm’s situation with respect to the incidence of clinical mastitis due to E. coli with its corresponding economic losses will play a role.
- Second, the market price of this vaccine in your area.
- Finally, the level of improvement that we obtain once we have implemented the vaccination plan.
Assesment of the initial situation
As mentioned above, the first point to ascertain is the udder health of a farm and how much money clinical mastitis is costing.
This is not an easy figure to obtain, as many factors are involved. In other words, a hyper-acute mastitis due to E. coli in an animal’s first lactation 3 days post-partum and leading to the death of the animal is not going to have the same cost as a mild case in an animal in its fourth lactation that can be easily cured, or the animal slaughtered to recover its meat value. However, even if it is a difficult value to obtain, we must not cease attempting to estimate it.
“Clinical mastitis involves both direct and indirect costs”
Direct costs:
- Treatment cost.
- The loss due to discarded milk.
- The loss of future production (compared to animals that have not suffered mastitis).
- Increase in replacement of animals.
- Veterinary services (if necessary).
- The increase in working hours.
Within indirect costs:
- The increase in tank somatic cell count (with the possible loss of bonuses or even extra penalties on the price of milk).
- Fat and protein reduction in milk.
- Increased risk of residues in milk.
- The negative impact on reproductive performance. The final factor carries greater economic impact than we can imagine.
If we review the scientific literature, we shall find an enormous degree of variability in the calculation of the economic impact of clinical mastitis. Dr Alfonso Lago made a compilation of the studies in this regard up to 2010, which can be seen in the following table:
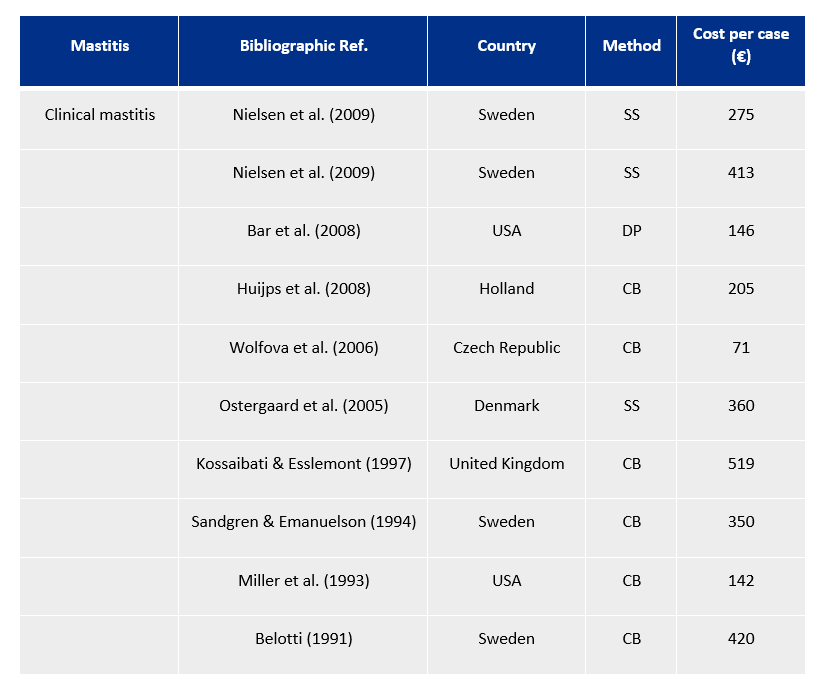
This enormous variability between studies is due to many factors relating to the animal itself and the agent causing mastitis. For example, mastitis at the start of lactation will generally have a greater economic impact than a case at the end of lactation; or the number of an animal’s lactational periods, or the number of cases of clinical mastitis during lactation, in the case of mild, severe or hyper-acute mastitis, will also have very different economic consequences, etc.
The pathogen involved also makes a significant difference. E. coli or Klebsiella mastitis usually leads to greater production losses than CNS mastitis, for example. Furthermore, pathogens that do not respond to treatments, such as Mycoplasma bovis, Prototheca or Trueperella, also have very significant associated costs.
References:
1. Allore, H.G. et al. Partial budget of the discounted annual benefit of mastitis control strategies. J. Dairy Sci., vol 81, num 8, 1998
2. Bar, D. et al. The cost of generic clinical mastitis in dairy cows as estimated by using dynamic programming. J. Dairy Sci., vol 91, 2008
3. Bar, D. et al. Use of a dynamic programming model to estimate the value of clinical mastitis treatment and prevention options utilized by dairy producers. Agricultural Systems 99, 6-12. 2009
4. Nickerson S.C. and Oliver S.P. Review: How well have Unites States dairy producers adopted mastitis-control technologies for reducing herd somatic cell counts and improving milk quality? The Professional Animal Scientist 30: 115-124. 2014
5. Baucells, J. El coste económico de la mastitis en vacuno lechero: Evaluación y cálculo. BA (Boletín ANEMBE), num 109.
6. Bradley, A.J., An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimen under field conditions in the United Kingdom. J. Dairy Sci. vol 98, 2015
7. DeGraves F.J., Fetrow J. Economics of mastitis and mastitis control. Veterinary Clinics of North America: Food animal practice. Vol 9, num 3, 1993
8. Hogan, J.S. et al. Efficacy of an Escherichia coli J5 bacterin administered to primigravid heifers. J. Dairy Sci. vol 82, 1999
9. Kessels, J.A. et al. Economic comparison of common treatment protocols and J5 vaccination for clinical mastitis in dairy herds using optimized culling decisions. J. Dairy Sci., vol 99, 2016
10. Vargas, R.T. et al. Partial budget analysis of prepartum antimicrobial therapy and Escherichia coli J5 vaccination of dairy heifers and their effect on milk production nd milk quality parameters. Pesq. Vet. Bras vol 36, 2016.
11. Wilson D.J. and González R. Vaccination strategies for reducing clinical severity of coliform mastitis. Vet Clin Food anim 19: 187-197. 2003
12. Lago, A. et al. A partial budget analysis to estimate the economics of a mastitis vaccination program. Poster format. 2012
13. Hertl, J.A. et al. Pathogen-specific effects on milk yield in repeated clinical mastitis episodes in Holstein dairy cows. J. Daisy Sci 97: 1465-1480. 2014
14. Rollin, E. et al. The cost of clinical mastitis in the first 30 days in lactation: An economic modelling tool. Preventive Vet Med 122: 257-264. 2015


