Substances to be used in teat dips
In this post entry we will look at the differences between the actives and realise that there are many similarities e.g. often being inactivated by organic matter. On the other hand we will see that there is an optimum concentration for some products and that the active is not actually to blame for product failure but rather the focus to attention of the mixing, application, etc. of the teat dip by the milker is.
A) Iodine
- Broad-spectrum properties (bacteria, fungi and some spores).
- Mechanism of action (MOA) is an oxidising killing method of the free iodine molecule. The oxidising agent removes electrons from the cell wall/membrane. The removal of amino acids in the protein production and maintenance of the wall/membrane.
- Some iodine compounds don’t contain a free iodine molecule (no oxidising effect), but rather alters the stability (pH: 5 – 6) of the environment.
- Iodine concentrations range of 0,025% to 0,3%, but there is an optimum concentration as the different concentrations have nearly the same amount of free iodine present (Böhm, F. et al., 2017a,b). Going above this to achieve a “better” disinfecting effect will not have the expected results. The concentrations are normally 0,5% for pre milking teat dips and 1% for post milking teat dips.
- Negative properties: irritating to the skin (certain weather conditions or with pre-existing skin damage). could mean one has to look at the other properties of the dip (viscosity, film production and high levels of emollients).
- EU regulations (human health laws: toxicity and residual levels that are in the milk).

Same substance kept in different conditions, (like iodine reacts to light). Left one, is from a bottle opened some time ago and not properly storage, right one is just new bottle. The of the colour loss indicates a minor content of iodine.
B) Chlorhexidine (Biguanides)
- Second most common teat dip active.
- Many positive benefits for the skin (neutral pH and non-irritant effects).
- Not a broad – spectrum effect.
- Different concentrations (0.35 ‐ 0.5%) – lower conc. is bacteriostatic and higher conc. is bactericidal. Research shows a conc. ≥ 0.5% is needed to be efficacious.
- MOA is the chemical disrupting the membranes in the cell walls of gram positive bacteria, but is ineffective against gram negative bacteria like Serratia and Pseudomonas.
- Could be favoured as a post milking teat dip with teats that are badly damaged and its residual effect, but it does not rule out the product as a pre milking teat dip. (Gilbert, P. and Moore, L.E., 2005).
C) Acidified Sodium Chlorite (ASC): e.g. Chlorine dioxide
- Fast acting broad-spectrum (inclusive of yeasts and viruses).
- MOA is an oxidising killing mechanism and is effective from conc. of 0,32%.
- Used as both a pre and post milking teat dip.
- Many positive benefits for the skin (exfoliation or hydration).
- Works well with high levels of organic matter.
- Two part dip [sodium chlorite and acid (lactic acid)] that needs to be mixed each time.
- Negative properties: Short shelf life once mixed, conc. varies in the same container with use over time if not mixed properly before use each time.
D) Hydrogen peroxide
- Broad-spectrum properties.
- MOA is an oxidising killing mechanism at concentrations of 0,5% to 1%.
- Mixed solutions have synergistic effects in disinfection e.g. Lactic acid.
- Good foaming action – extended contact time, volume saving, etc.
- Many positive benefit for the skin health (exfoliation of outer skin layer).
- Negative properties: short shelf life and the product is sensitive to sun light and extreme temperatures. The active is not as effective under high organic matter contamination.
E) Organic acids: e.g. Lactic acid
- MOA is the disruption of the cell membrane of the bacteria.
- Mixed solutions to have synergistic effects in disinfection.
- Product for the future as regulations get stricter.
- Many positive benefits associated with this product e.g. good skin care, quick acting, highly compatible with emollients, etc.
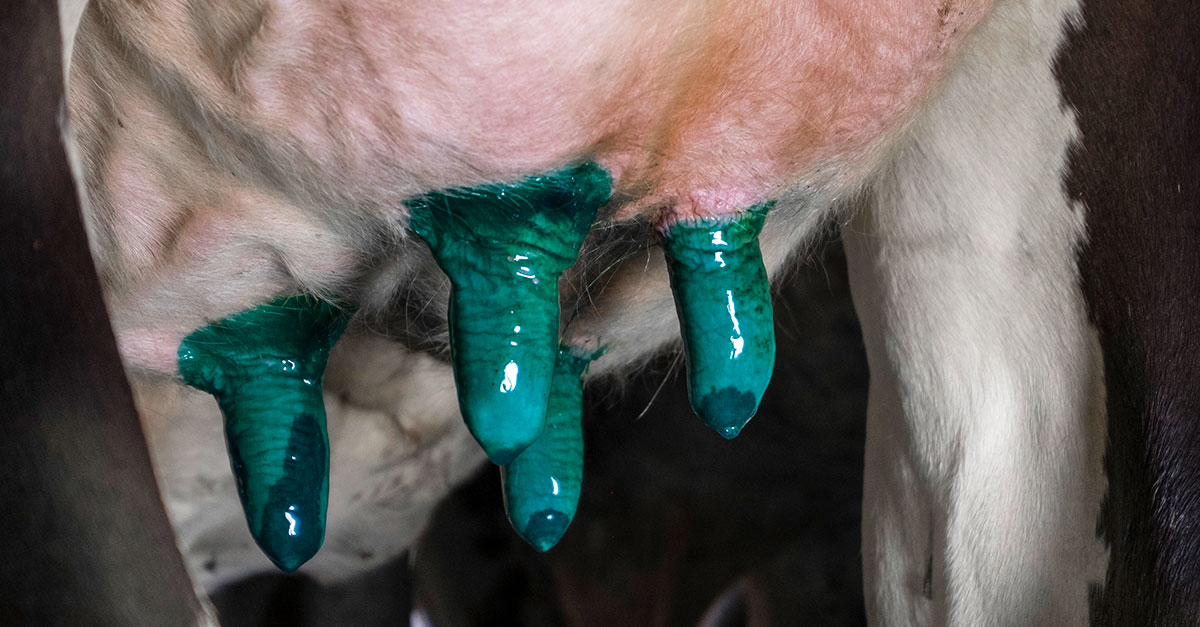
Colour of the teat dip is important to help checking proper coverage is done. Like in the case of acid lactic, that is coloured with green pigments purposely.
F) Phenols/Alcohols
- Less common in areas of warmer climates (dries quickly and reduces skin damage with freezing issues).
- EU has removed phenols in teat dips due to carcinogenic properties.
G) Quaternary Ammonium Compounds (QACs)
- The MOA is the perturbation of lipid bilayer membranes and denaturing of cell proteins resulting in loss of osmoregulatory capability and a leakage of potassium ions and protons.
- To obtain a broad-spectrum the QAC must be mixed with another compound.
- This active is more effective against gram positives and fungi, with limited efficacy against gram negative bacteria. Not effective against Serratia and Pseudomonas bacteria (Gilbert, P. and Moore, L.E., 2005).
- EU has strict restrictions due to a high level of milk contamination.
The take home message here is to use the best that you can afford, and make the decision on what the cow’s teats and udder are telling you. The formulation and application of the teat dip is of utmost importance when evaluating the teat dips.
Author:



6 Comments
Very useful information, I only would add the water solubility of the teat dip products, how easy is it to remove them during the pre-milking udder washing process, a very important factor to be considered to minimize the risk of milk contamination.
Totally agree, thanks for your comment!
Thanks for leaving a comment, next series of posts about disinfectants, we will take this into account.
Regards,
As there are queries over the efficacy of chlorhexidine against gram negative bacteria (although I am aware you have authored articles stating that the active ingredient and concentrations are not necessarily 100% responsible for efficacy) what would be the recommended product for environmental gram negative bacteria (such as psedomonas, klebsiella, serratia, enterococcus etc)? Taking into consideration of the restrictions on the use of chlorine dioxide, QACs and worries around iodine residues.
Hi, Thanks for your comment! 🙂
You are completely right that the active ingredient and its concentration are not an accurate measure of a disinfectant efficacy. Other components of the formulation will impact the efficacy, like surfactants for example, that are not listed on the materials, making the process of comparison of the product very difficult, if not impossible, based on label, technical sheet and safety data sheet. Even the claims can be a topic of discussion, as there are several test norms, with different protocols, so are not comparable. Finally, the European Biocidal Products Regulation has come to make things clearer – https://echa.europa.eu/ . The active substances are being reviewed and then products are being submitted. On the process they will be subject to new efficacy standards that mimic more approximately the use conditions, so that will be very beneficial. So far, the process is completed for iodine and ongoing for lactic acid, hydrogen peroxide and peracetic acid.
Chlorine dioxide is still on evaluation as active substance and due to generation in situ, the conditions of generation, the release and stability are difficult questions, that remain unanswered. Iodine also poses limitations of the residues, being now recommended only before milking or only after milking, not on both occasions. Iodine products with the new European registration are the products with the most documented and verifiable claim, so they inspire trust. Traditionally lactic acid products are considered to be good solution for gram negative bacteria, although there is a big variation between formulations from different companies. Lactic acid, hydrogen peroxide and peracetic acid pose low risk of milk contamination as they are (or they by-products) “normal” milk components.
This makes them good candidates, specially for pre-dipping, but once we have these products with European authorization we will be even more confident in their conditions of use.
Aditionally, post dipping has very limited impact on the control of environmental pathogens. Pre dip can have a more important role, but it should not be regarded as a silver bullet as other factors play also a critical role (cow hygiene score, bedding, immune system, etc) in achieving a satisfactory control of environmental mastitis.
As always, take into consideration that this is a recommendation done online, so that means the basic point to have good recommendations is to be able to visit the farm, and that was not done. All recommendations should be checked in every particular case.
Ok
Comments are closed.